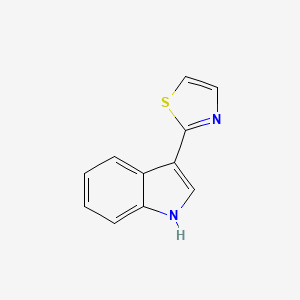


3-(1,3-thiazol-2-yl)-1H-indole
CAS No.: 135531-86-1
- Molecular Formula: C₁₁H₈N₂S
- Molecular Weight: 200.26 g/mol
Chemical type
- Indole-type phytoalexin (indolic phytoalexins/alkaloids)
- Indole phytoalexin (Plant-derived secondary metabolite)
Key properties
- Synthesized from tryptophan via indole-3-acetaldoxime (IAOx)
- Broad antimicrobial activity against fungi and bacteria
- Induced by pathogen-triggered immunity (PTI/ETI)
- Regulated by WRKY TFs and SA/JA signaling
- Antiproliferative and cancer chemopreventive effects
- Induces oxidative stress (Reactive Oxygen Species - ROS) in cancer cells
- Promotes lysosomal membrane permeabilization (LMP) and cathepsin D (CD) release
- Selectively more cytotoxic towards aggressive/metastatic prostate cancer cell lines (e.g., C4-2, ARCaPM) compared to less aggressive ones (e.g., LNCaP, ARCaPE)
- Antimicrobial defense in Arabidopsis and crucifers
- Inhibits pathogen growth and contributes to innate immunity
- Potential novel therapeutic agent for the treatment of metastatic prostate cancer
- Research tool for studying ROS-induced and lysosome-mediated apoptosis pathways
Classification by use
- Phytoalexins used in fungal and bacterial resistance
- Inducible secondary metabolites for biotic stress response
- Chemicals with potential therapeutic use in cancer treatment
- Chemicals used in biological/mechanistic research (apoptosis, oxidative stress)
- [1] Redefining phytoalexins as engineered defenses for plant disease resistance, Current Plant Biology, Volume 45, January 2026, 100577
- [2] Camalexin-Induced Apoptosis in Prostate Cancer Cells Involves Alterations of Expression and Activity of Lysosomal Protease Cathepsin D, Molecules, 2014, 19(4), 3988-4005