Your Location:Home > Products > Organic Raw Materials > Methyl 2-phenylacetate


CasNo: 101-41-7
MF: C9H10O2
Appearance: colourless liquid
|
Description |
Methyl phenylacetate is an organic compound that is a clear, colorless liquid and the methyl ester of phenylacetic acid. It has the structural formula C6H5CH2COOCH3 and is slightly soluble in water but highly soluble in most organic solvents. |
|
Synthesis and Preparation |
Methyl phenylacetate, an organic compound with the structural formula C6H5CH2COOCH3, can be synthesized through hydrolysis and esterification from phenylacetonitrile. Methyl phenylacetate can also be produced from benzyl cyanide through acid hydrolysis in the presence of methanol. Benzyl cyanide is derived from benzyl chloride, an industrial chemical. |
|
Biochem/physiol Actions |
Methyl phenylacetate is an organic compound with a strong honey-like odor that has many uses. Methyl phenylacetate is the starting material in manufacture of synthetic perfumes. |
|
Safety Profile |
Moderately toxic by ingestion and skin contact. A skin irritant. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS. |
|
Definition |
ChEBI: Methyl benzeneacetate is a member of benzenes. |
|
Aroma threshold values |
Detection: 25 ppb |
| Production Plant | DEMEI ELECTRONIC COMMERCE FIRM was established in 2024 and is located in Yiwu, Zhejiang Province. Although it was established not long ago, it has more than ten years of industry experience. The affiliated factory covers an area of 7,000 square meters. In addition, it also has its own excellent reserve of talents. Our high-tech R&D team, workshop production and sales R&D personnel have exceeded 200 people. In recent years, our products have also been exported to the UK, Germany, Australia, the Netherlands, Poland, Russia, Kazakhstan, the Philippines, Malaysia, Singapore and many other regions. |
|
Who Evaluation |
Evaluation year: 2002 |
InChI:InChI=1/C9H10O2/c1-11-9(10)7-8-5-3-2-4-6-8/h2-6H,7H2,1H3
Forty one microorganisms belonging to different taxonomical groups were used to carry out the enantioselective reduction of methyl benzoylformate to afford the corresponding (R)-methyl mandelate, with moderate to high ee. In contrast, the monooxygenase enzyme in Helminthosporium sp. CIOC3.3316 catalyzed the hydroxylation of methyl 2-phenylacetate to (S)-methyl mandelate. This combination of oxidation and reduction biotransformations thus provides a method for preparing the enantiomers of chiral α-hydroxy acid derivatives.
Sacrificial anode-free efficient electro...
The new phosphinite and phosphonite comp...
Many kinds of aromatic nitriles have bee...

diazomethane
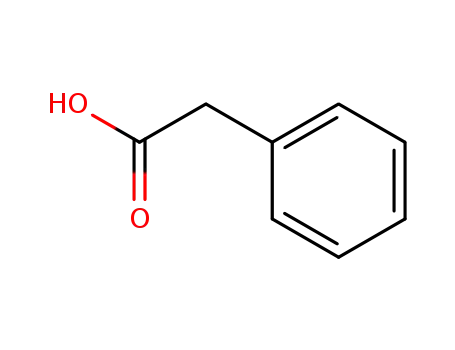
phenylacetic acid
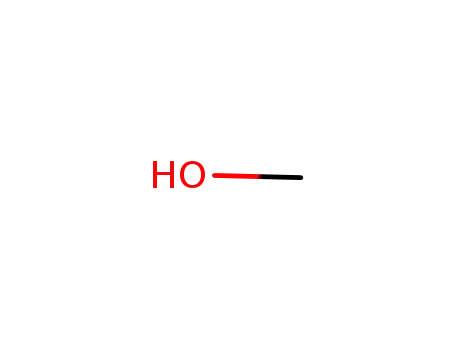
methanol
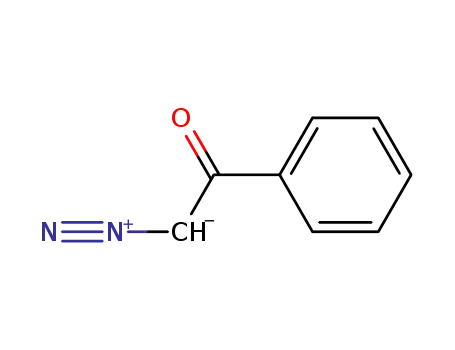
2-diazo-acetophenone
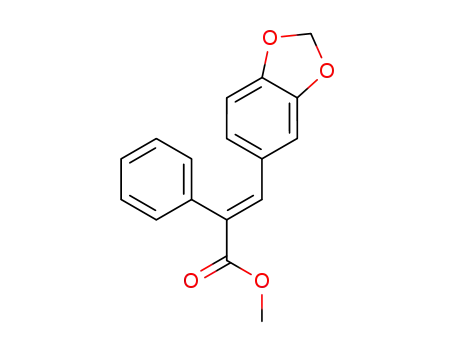
methyl (E)-3-(benzo[d][1,3]dioxol-6-yl)-2-phenylacrylate
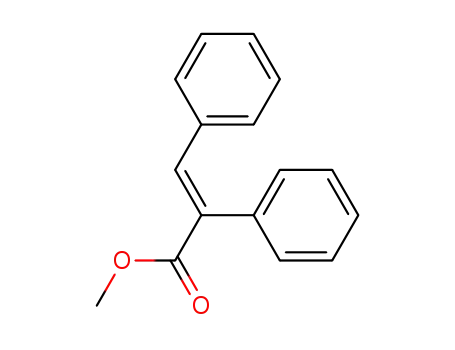
methyl (E)-2,3-diphenyl-2-propenoate
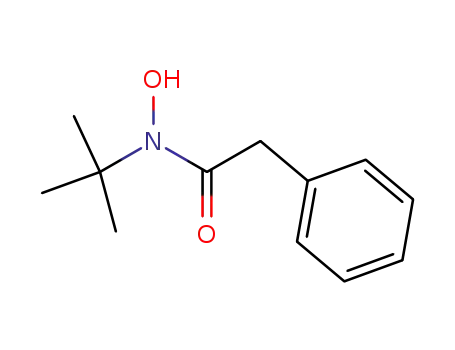
N-phenylacetyl-N-tert-butylhydroxylamine
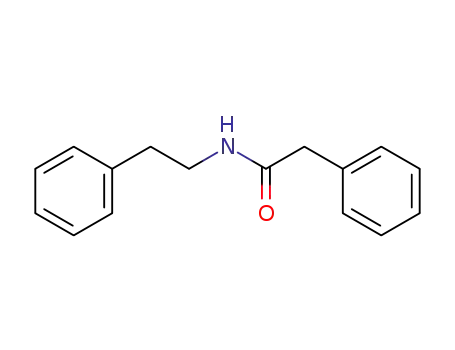
N-phenethyl-2-phenylacetamide